Studying macrophages is old hand: in general we know M1 macrophages damage tissue and M2 repair them. But can we parse them into smaller groups? Where do they come from? Do they settle in before birth or develop as an adult?
“They are of underappreciated importance,” says Jillian Macklin, one of the first recipients of a Ted Rogers Centre for Heart Research Education Fund award. “There could be many subsets that are performing many different roles, far beyond the classic M1 and M2. We need to find the differences. So far, Slava has identified two origins of macrophages: embryonic, which we are born with, and adult, which develop as we mature.”
For the heart, these differences are of enormous importance. Macklin’s supervisor, Dr. Slava Epelman, a Toronto General Research Institute scientist, suspects that embryonic macrophages are key to repairing the heart.
Macrophages after a heart attack
Heart attacks deplete the heart’s immune cells, triggering a huge infiltration of monocytes, which are white blood cells. Prior to that event, research has revealed a balance of embryonic and adult macrophages. Afterward, that wave of monocytes differentiate into damaging adult macrophages with a loss of the embryonic macrophages. Much research is underway to see what can be done to protect the heart from its own immune system.
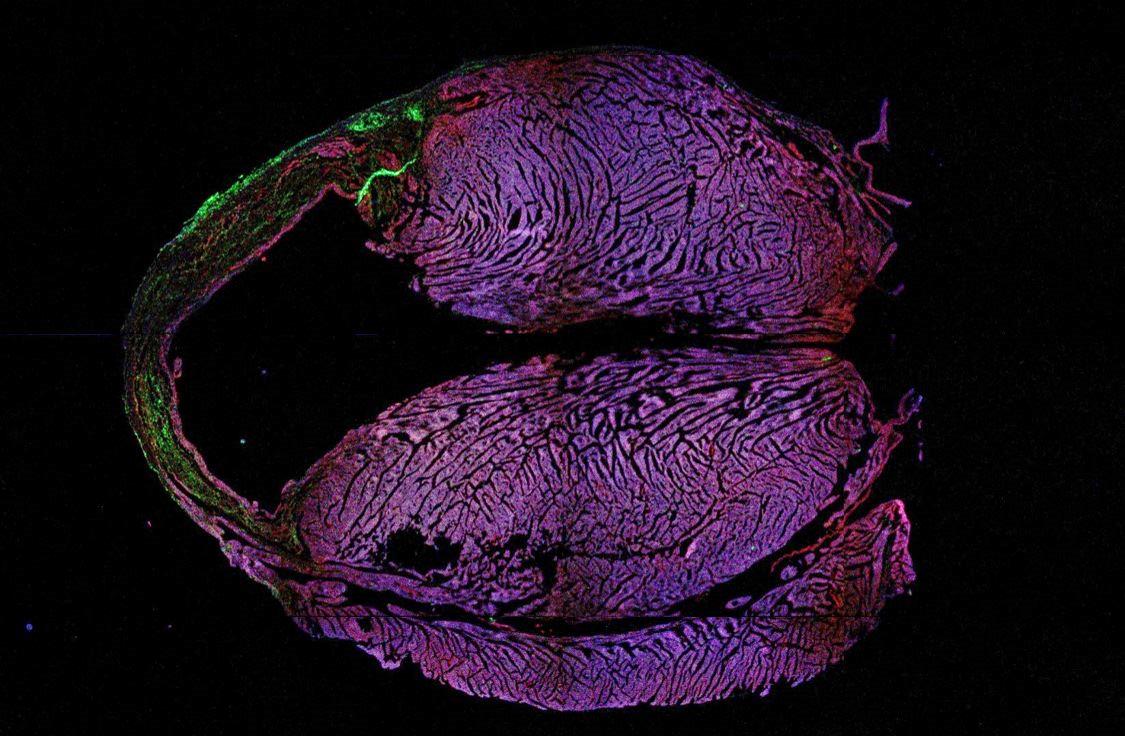
Macklin is studying mice that have had a heart attack. With Dr. Epelman’s guidance, she is targeting the embryonic macrophages. Dr. Epelman has developed novel genetic tools that allow you to identify and track these cells using fluorescent tags, followed by depleting them.
“If I’m able to confidently deplete the embryonic macrophages and see functional differences in the heart, that will be a big success,” Macklin says.
If she finds that embryonic macrophages are indeed reparative after a heart attack, it could spark more focused research. How can we stimulate these and make them repair the heart after an event? Could we cause them to proliferate in the body? Could we inject patients with embryonic macrophages? And the big question: could they help the heart return to normal size and function after a major cardiac event?
Macklin says the TRCHR fund helped open up a community of investigators, particularly University of Toronto bioengineers experienced in stem cell work. “Heart failure in itself does not involve just biochemistry or just immunology. It involves them all. So that’s why you need experts from every field to collaborate,” she says.
Jillian Macklin’s project is called “Different origins of cardiac macrophage subsets and their role in ischemic injury.” For more information on Dr. Slava Epelman’s research, click here.