In response to myocardial infarction, the adult heart undergoes a wound healing process that shows remarkable parallels with wound repair in other tissues, including the epidermis. Both in heart and skin, wound repair causes the formation of scar tissue. Scars limit cardiac output and can lead to heart failure.
Unlike adults, embryos have a striking ability to repair wounds with no scarring. Embryonic wound repair is driven by polarized cytoskeletal rearrangements and contractile force generation at the wound edge. However, the molecular and physical mechanisms that trigger and organize cell behaviours for scarless embryonic wound repair are not well understood.
Drosophila melanogaster
Our lab in the Ted Rogers Centre for Heart Research investigates the mechanisms by which tissues form and repair in embryos. In particular, we exploit the power of quantitative live imaging, biophysical methods and molecular genetics to understand how wounds are repaired in embryos of the fruit fly, Drosophila melanogaster.
We image the wound repair process live, taking advantage of transgenic animals expressing fluorescently-tagged proteins of interest. We develop and apply image analysis and machine learning algorithms to measure the localization and dynamics of different molecular markers during embryonic wound repair. With laser-based and optogenetic approaches, we locally quantify, manipulate and investigate the role of mechanical forces in the wound healing process. We screen for factors that disrupt or enhance wound repair in the embryo using pharmacological and genetic perturbation. Finally, we build mathematical models that integrate cell mechanics and molecular dynamics to interpret our data and develop new hypotheses that can be further tested in vivo.
Our work has already shed new light into the mechanisms of embryonic wound repair. We found that polarized trafficking within the cells around the wound is critical for the cytoskeletal rearrangements that drive scarless repair. We are now beginning to identify the molecular signals that polarize the trafficking machinery, and the cargo that is transported. In addition, we found that force generation around the wound is not uniform, but patterned, and that the pattern of contractile forces is critical for rapid wound healing. The mechanisms of embryonic wound repair are conserved across tissues and species.
Therefore, our findings will have direct implications to understand scarless tissue repair, and may lead to the development of novel strategies to prevent myocardial scar formation and heart failure.
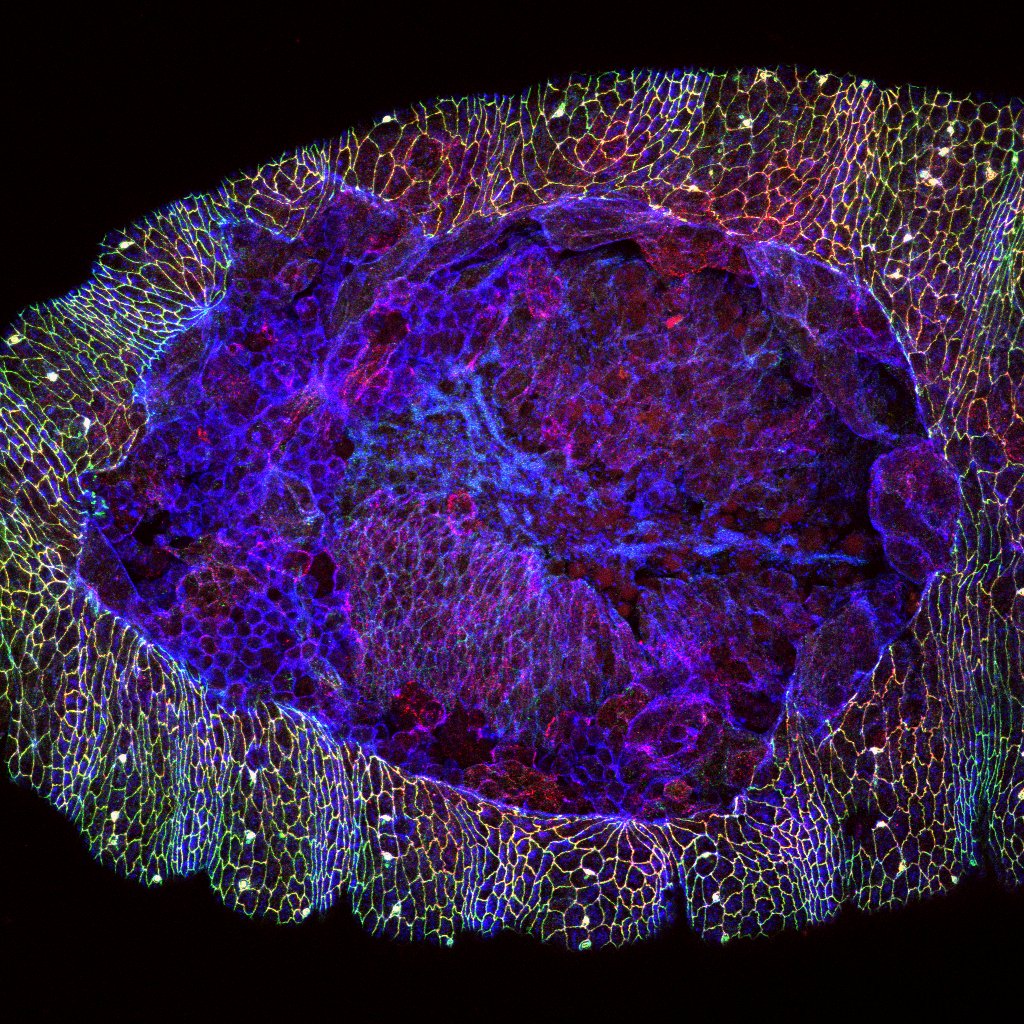
Image: A wound in the epidermis of a fruit fly embryo. (Courtesy Rodrigo Fernandez-Gonzalez)